New trends in Pathology (Part 1)
Written by Ilgar Guseinov on 29.11.2022
Many definitions of «Digital pathology» can be found in articles and official societies, but each one is right and wrong at the same time. Some are too concise and do not allow you to see the full picture behind them, while others see this field only as a tool in the service of an already existing branch of medicine. Therefore, let us limit ourselves for the time being to the following definition, so that it will be clear to those who are more interested in computer science, and the specialists of this field will not get annoyed.
Digital pathology is the practice of pathology using high-resolution digital images rather than a traditional microscope's eyepiece to examine tissue or fluid samples.
In this and the following articles, we will expand on that definition and explain how med4PAN project improves the level of care with 5G technologies.
But before we get to the digital images themselves, let's look at the infrastructure of a typical digital pathology lab. Of course, we can start right away with digital imaging and processing, but it won't make that much sense on its own.
Digital Workflow
In any pathology lab, whether digital or traditional, the same things happen, but digitalization puts its mark on every step.
Accepting a specimen
Despite the fact that this stage sounds quite simple, but a lot depends on its quality performance. The objectives of this stage are to minimize pre-analytical factors that can affect the quality of the slides and the image, and therefore the result. The intactness of the container in which the sample arrived, the adequacy of the information in the covering documents, the presence of contact information and the consistency of the documents and the contents is very important information that should be clarified before entering this data into the laboratory information system (LIS). Here it is also necessary to get rid of all paper documents and enter all data (as far as possible) into the LIS. After entering all the data, the sample receives a unique code and QR, otherwise it will not be possible to continue to pass through the laboratory.
Figure 1. Comparison of modern labels and handwritten identifiers of cassettes and slides. [1]
Figure 2. Examples of barcoded paper requisition, specimen container, cassette engraver label and slide label. [2]
Learn more about barcoding tracking system here: https://www.pathologyoutlines.com/topic/informaticsbarcodingtracking.html
A laboratory information system (LIS) is a software application or database that is used to manage, store, and analyze data generated in a laboratory setting. It is a critical component of a laboratory's operations, as it helps to ensure the accuracy, integrity, and security of the data that is collected and analyzed.
Some of the key functions of a laboratory information system include:
Data management: A LIS can be used to store, organize, and manage all of the data generated in a laboratory, including test results, patient information, instrument calibration records, and more. This can help to ensure that data is accurately recorded and easily accessible for analysis and reporting.
Quality control: A LIS can be used to implement quality control measures, such as automated checks for errors or out-of-range values, to help ensure the accuracy and reliability of the data.
Reporting: A LIS can generate reports and other types of data outputs that can be used for a variety of purposes, such as quality assurance, regulatory compliance, or research and development.
Integration: A LIS can be integrated with other systems and software, such as electronic health records, to facilitate the exchange of information and improve workflow.
Overall, a laboratory information system can greatly improve the efficiency and effectiveness of a laboratory's operations and is an essential tool for any organization that relies on data-driven decision making.
Learn more about LIS here: https://www.youtube.com/watch?v=uVz25xKRcYw
Allocation and prioritization of specimens
Then the registered and labeled samples are delivered to the laboratory where they can be sorted (e.g., by complexity) or prioritized by urgency, and then transferred to the section.
Macroscopic (gross) examination
The process of the section (macroscopic examination) is aimed at describing the macro specimen, finding and highlighting the areas of interest that will be placed further in the cassettes. This is one of the key stages of the whole process. Mistakes at this stage, can lead to the loss of areas that can answer important questions.
Figure 3. An example of a grossing station. 1 – camera; 2 - touch screen monitor; 3 – microphone; 4 - section holding are; 5- table and component control pedals [3]
Compared to the traditional workstation of a pathologist, the modern workstation has a number of differences. After receiving a specimen, a specialist scans the QR code using a reader, and the automatically registered case in LIS is shown on the monitor. A camera mounted over the workstation allows taking photos and fixing the parameters of the sample. This data is immediately uploaded to a local server and attached to the current case in the LIS. During the macroscopic examination, a microphone can be used to record the examination protocol.
An example of the macroscopic examination step can be seen here https://www.youtube.com/watch?v=W-YS87ZJHM0
Processing
The processing step in the laboratory is not much different from that in a normal laboratory. But here the processes are also highly automated according to the guidelines.
Embedding
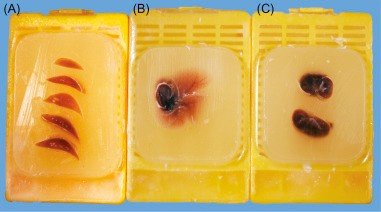
After the necessary measurements and descriptions are made, the specimens are placed in cassettes and filled with paraffin for fixation. After cooling, a paraffin (histological) block is obtained (fig.4). In this way the specimen can be preserved for a long time.
Figure 4. Paraffin-embedded tissue [4]
See this clearly in the video: https://www.youtube.com/watch?v=xIyXA3c3oxU
Cutting
In order to obtain slides from the tissues fixed in paraffin, it is required to cut it into thin slices. This is done with a machine called a microtome (see video). After obtaining a successful slice, it is applied to a slide, fixed, and sent for staining.
https://www.youtube.com/watch?v=XDoTLJ3ZXtY
Staining and mounting
To be able to visualize and evaluate tissue microstructures better, it is necessary to stain them. Routinely H&E stain (Hematoxylin and Eosin) is used - Hematoxylin stains structures containing DNA, RNA (cell nucleus, ribosomes and RNA-rich parts of cytoplasm), and eosinophil stains cytoplasm and its structures. But in addition to this there are a large number of stains for the identification of different cell structures. Today the staining process is fully automated.
https://www.youtube.com/watch?v=6-WOgY5-P04
Now the slides are ready for scanning and examining, but more on that in the next post!
References:
[1] Brown, R. W., Speranza, V. D., Alvarez, J. O., Eisen, R. N., Frishberg, D. P., Rosai, J., ... & Thomas, N. E. (2015). Uniform labeling of blocks and slides in surgical pathology: Guideline from the college of American Pathologists Pathology and Laboratory Quality Center and the National Society for Histotechnology. Archives of Pathology & Laboratory Medicine, 139(12), 1515-1524
[2] Absar, F., & Prichard, W. (2022, March 7). Barcoding and tracking. https://www.pathologyoutlines.com/topic/informaticsbarcodingtracking.html
[3] https://www.milestonemedsrl.com
[4] Treuting, P. M., Dintzis, S., & Montine, K. S. (Eds.). (2017). Comparative anatomy and histology: a mouse, rat, and human atlas. Academic Press.